SUPAC guidelines are FDA guidelines for Scale-Up and Post-Approval Changes for manufacturing of drugs
Ø SUPAC-IR: Immediate-Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation
Ø SUPAC-MR: Modified Release Solid Oral Dosage Forms Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation
Ø SUPAC-SS: Nonsterile Semisolid Dosage Forms; Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation
SUPAC-IR: Immediate-Release Solid Oral Dosage Forms:
Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation
I. PURPOSE OF GUIDANCE
This guidance provides recommendations to sponsors of new drug applications (NDA's), abbreviated new drug applications (ANDA's), and abbreviated antibiotic applications (AADA's) who intend, during the postapproval period, to change: 1) the components or composition; 2) the site of manufacture; 3) the scale-up/scale-down of manufacture; and/or 4) the manufacturing (process and equipment) of an immediate release oral formulation. This guidance is the result of: 1) a workshop on the scale-up of immediate release drug products conducted by the American Association of Pharmaceutical Scientists in conjunction with the United States Pharmacopoeial Convention and the Food and Drug Administration (FDA); 2) research conducted by the University of Maryland at Baltimore on the chemistry, manufacturing and controls of immediate release drug products under the FDA/University of Maryland Manufacturing Research Contract; 3) the drug categorization research conducted at the University of Michigan and the University of Uppsala on the permeability of drug substances; and 4) the Scale Up and Post Approval Changes (SUPAC) Task Force which was established by the Center for Drug Evaluation and Research (CDER) Chemistry, Manufacturing and Controls Coordinating Committee to develop guidance on scale-up and other post approval changes.
The guidance defines: 1) levels of change; 2) recommended chemistry, manufacturing, and controls tests for each level of change; 3) in vitro dissolutiontests and/or in vivo bioequivalence tests for each level of change; and 4)documentation that should support the change. For those changes filed in a “changes being effected supplement” [21 CFR 314.70(c)], the FDA may, after a review of the supplemental information, decide that the changes are not
approvable. This guidance thus sets forth application information that should be provided to CDER to assure continuing product quality and performance characteristics of an immediate release solid oral dose formulation for specified postapproval changes. This guidance does not comment on or otherwise affect compliance/inspection documentation that has been defined by CDER’s Office of Compliance or FDA’s Office of Regulatory Affairs. This guidance does not affect any postapproval changes other than the ones specified. For changes not addressed in this guidance, or for multiple changes submitted at one time or over a short period of time, or where the number of batches needed for stability testing is not specified, sponsors should contact the appropriate CDER review division or consult other CDER guidances/guidelines to obtain information about tests and application documentation.
COMPONENTS AND COMPOSITION
This section of the guidance focuses on changes in excipients in the drug product. Changes in the amount of drug substance are not addressed by this guidance. Changes in components or composition that have the effect of adding a new excipient or deleting an excipient are defined at Level 3 (defined below), except as described below.
A. Level 1 Changes
1. Definition of Level:Level 1 changes are those that are unlikely to have any detectable impact on formulation quality and performance.
Examples:
a. Deletion or partial deletion of an ingredient intended to affect the color or flavor of the drug product; or change in the ingredient of the printing ink to another approved ingredient.
b. Changes in excipients, expressed as percentage (w/w) of total formulation, less than or equal to the following percent ranges:
These percentages are based on the assumption that the drug substance in the product is formulated to 100% of label/potency. The total additive effect of all excipient changes should not be more than 5%. (Example: In a product consisting of active ingredient A, lactose, microcrystalline cellulose and magnesium stearate, the lactose and microcrystalline cellulose should not vary by more than an absolute total of 5% (e.g. lactose increases 2.5% and microcrystalline cellulose decreases by 2.5%) relative to the target dosage form weight if it is to stay within the Level 1 range).
2. Test Documentation
a. Chemistry Documentation:Application/compendial release requirements and stability testing.
Stability testing: one batch on long-term stability data reported in annual report.
b. Dissolution Documentation :None beyond application/compendial requirements.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation:Annual report (all information including long-term stability data).
B. Level 2 Changes
1. Definition of Level:Level 2 changes are those that could have a significant impact on formulation quality and performance. Tests and filing documentation for a Level 2 change vary depending on three factors: therapeutic range, solubility, and permeability.
Therapeutic range is defined as either narrow or non-narrow. A list of narrow therapeutic range drugs is provided in Appendix A. Drug solubility and drug permeability are defined as either low or high. Solubility is calculated based on the minimum concentration of drug, milligram/milliliter (mg/mL), in the largest dosage strength, determined in the physiological pH range (pH 1 to 8) and temperature (37 + 0.5oC). High solubility drugs are those with a dose/solubility volume of less than or equal to 250 mL. (Example: Compound A has as its lowest solubility at 37 + 0.5oC, 1.0 mg/mL at pH 7, and is available in 100 mg, 200 mg and 400 mg strengths. This drug would be considered a low solubility drug as its dose/solubility volume is greater than 250 mL (400 mg/1.0 mg/mL=400 mL). Permeability (Pe, centimeter per second) is defined as the effective human jejunal wall permeability of a drug and includes an apparent resistance to mass transport to the intestinal membrane. High permeability drugs are generally those
with an extent of absorption greater than 90% in the absence of documented instability in the gastrointestinal tract, or those whose permeability attributes have been determined experimentally).
Examples:
a. Change in the technical grade of an excipient. (Example:Avicel PH102 vs. Avicel PH200.)
b. Changes in excipients, expressed as percent (w/w) of total formulation, greater than those listed above for a Level 1 change but less than or equal to the following percent ranges (which represent a two fold increase over Level 1 changes):
These percentages are based on the assumption that the drug substance in the drug product is formulated to 100% of label/potency. The total additive effect of all excipient changes should not change by more than 10%. The components (active and excipients) in the formulation should have numerical targets that represent the nominal composition of the product on which any future changes in the composition of the product are to be based. Allowable changes in the composition should be based on the approved target composition and not on the composition based on previous Level 1 or Level 2 changes.
2. Test Documentation
a. Chemistry Documentation
Application/compendial release requirements and batch records.
Stability testing: 1 batch with 3 months accelerated stability data in supplement and 1 batch on long-term stability.
b. Dissolution Documentation
Case A: High Permeability, High Solubility Drugs Dissolution of 85% in 15 minutes in 900 mL of 0.1N HCl. If a drug product fails to meet this criterion, the applicant should perform the tests described for Case B or C
Case B: Low Permeability, High Solubility Drugs Multi-point dissolution profile should be performed in the application/compendial medium at 15, 30, 45, 60 and 120 minutes or until an asymptote is reached. The dissolution profile of the proposed and currently used product formulations should be similar.
Case C: High Permeability, Low Solubility Drugs Multi-point dissolution profiles should be performed in water, 0.1N HCl, and USP buffer media at pH 4.5, 6.5, and 7.5 (five separate profiles) for the proposed and currently accepted formulations. Adequate sampling should be performed at 15, 30, 45, 60, and 120 minutes until either 90% of drug from the drug product is dissolved or an asymptote is reached. A surfactant may be used, but only with appropriate justification. The dissolution profile of the proposed and currently used product formulations should be similar.
c. In Vivo Bioequivalence Documentation
None: if the situation does not meet the description in Case A, Case B or Case C, refer to Level 3 changes
3. Filing Documentation:Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
C. Level 3 Changes
1. Definition of Level:Level 3 changes are those that are likely to have a significant impact on formulation quality and performance. Tests and filing documentation vary depending on the following three factors: therapeutic range, solubility, and permeability.
Examples:
- Any qualitative and quantitative excipient changes to a narrow therapeutic drug beyond the ranges noted in Section III.A.1.b.
- All other drugs not meeting the dissolution criteria under Section III.B.2.b.
- Changes in the excipient ranges of low solubility, low permeability drugs beyond those listed in Section III.A.1.b.
- Changes in the excipient ranges of all drugs beyond those listed in Section III.B.1.b.
2. Test Documentation
a. Chemistry Documentation
Application/compendial release requirements and batch records.
Significant body of information available: One batch with three months accelerated stability data reported in supplement; one batch on long-term stability data reported in annual report.
Significant body of information not available: Up to three batches with three months accelerated stability data reported in supplement; one batch on long-term stability data reported in annual report.
b. Dissolution Documentation
Case B dissolution profile as described in Section III.B.2.b.
c. In Vivo Bioequivalence Documentation:Full bioequivalence study. The bioequivalence study may be waived with an acceptable in vivo/in vitro correlation has been verified.
3. Filing Documentation
Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
SITE CHANGES
Site changes consist of changes in location of the site of manufacture for both company-owned and contract manufacturing facilities and do not include any scale-up changes, changes in manufacturing (including process and/or equipment), or changes in components or composition. Scale-up is addressed in Section V of this guidance. New manufacturing locations should have a satisfactory current Good Manufacturing Practice (CGMP) inspection.
A. Level 1 Changes
1. Definition of Level
Level 1 changes consist of site changes within a single facility where the same equipment, standard operating procedures (SOP's), environmental conditions (e.g., temperature and humidity) and controls, and personnel common to both manufacturing sites are used, and where no changes are made to the manufacturing batch records, except for administrative information and the location of the facility. Common is defined as employees already working on the campus who have suitable experience with the manufacturing process.
2. Test Documentation
a. Chemistry Documentation:None beyond application/compendial release requirements.
b. Dissolution Documentation:None beyond application/compendial release requirements.
c. In Vivo Bioequivalence Documentation:None.
3. Filing Documentation:Annual report.
B. Level 2 Changes
1. Definition of Level:Level 2 changes consist of site changes within a contiguous campus, or between facilities in adjacent city blocks, where the same equipment, SOP's, environmental conditions (e.g.,temperature and humidity) and controls, and personnel common to both manufacturing sites are used, and where no changes are made to the manufacturing batch records, except for administrative information and the location of the facility.
2. Test Documentation
a. Chemistry Documentation:Location of new site and updated batch records. None beyond application/compendial release requirements.One batch on long-term stability data reported in annual report.
b. Dissolution Documentation:None beyond application/compendial release requirements.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation:Changes being effected supplement; annual report (longterm stability test data).
C. Level 3 Changes
1. Definition of Level:Level 3 changes consist of a change in manufacturing site to a different campus. A different campus is defined as one that is not on the same original contiguous site or where the facilities are not in adjacent city blocks. To qualify as a Level 3 change, the same equipment, SOP's, environmental conditions, and controls should be used in the manufacturing process at the new site, and no changes may be made to the manufacturing batch records except for administrative information, location and language translation, where needed.
2. Test Documentation:
a. Chemistry Documentation: Location of new site and updated batch records. Application/compendial release requirements.
Stability:
Significant body of data available:One batch with three months accelerated stability data reported in supplement; one batch on long-term stability data reported in annual report.
Significant body of data not available:Up to three batches with three months accelerated stability data reported in supplement; up to three batches on long- term stability data reported in annual report.
b.Dissolution Documentation
Case B: Multi-point dissolution profile should be performed in the application/compendial medium at 15, 30, 45, 60 and 120 minutes or until an asymptote is reached. The dissolution profile of the drug product at the current and proposed site should be similar.
c. In Vivo Bioequivalence Documentation: None.
CHANGES IN BATCH SIZE (SCALE-UP/SCALE-DOWN)
Postapproval changes in the size of a batch from the pivotal/pilot scale biobatch material to larger or smaller production batches call for submission of additional information in the application. Scale-down below 100,000 dosage units is not covered by this guidance. All scale-up changes should be properly validated and, where needed, inspected by appropriate agency personnel.
A.Level 1 Changes
1. Definition of Level:Change in batch size, up to and including a factor of 10 times the size of the pilot/biobatch, where: 1) the equipment used to produce the test batch(es) is of the same design and operating principles;
2) the batch(es) is (are) manufactured in full compliance with CGMP's; and 3) the same standard operating procedures (SOP's) and controls, as well as the same formulation and manufacturing procedures, are used on the test batch(es) and on the full-scale production batch(es).
2. Test Documentation
a. Chemistry Documentation
Application/compendial release requirements. Notification of change and submission of updated batch records in annual report.
One batch on long-term stability reported in annual report.
b. Dissolution Documentation
None beyond application/compendial release requirements.
c. In Vivo Bioequivalence:None.
3. Filing Documentation:Annual report (long-term stability data).
B. Level 2 Changes
1. Definition of Level
Changes in batch size beyond a factor of ten times the size of the pilot/biobatch, where: 1) the equipment used to produce the test batch(es) is of the same design and operating principles; 2) the batch(es) is (are) manufactured in full compliance with CGMP'S; and 3) the same SOP's and controls as well as the same formulation and manufacturing procedures are used on the test batch(es) and on the full-scale production batch(es).
2. Test Documentation
a. Chemistry Documentation:Application/compendial release requirements. Notification
of change and submission of updated batch records.
Stability testing: One batch with three months accelerated
stability data and one batch on long-term stability.
b. Dissolution Documentation: Case B testing.
c. In Vivo Bioequivalence:None.
3. Filing Documentation:Changes being effected supplement; annual report (long-term stability data).
MANUFACTURING
Manufacturing changes may affect both equipment used in the manufacturing process and the process itself.
A. Equipment
1. Level 1 Changes
a. Definition of Change
This category consists of: 1) change from non-automated or non-mechanical equipment to automated or mechanical
equipment to move ingredients; and 2) change to alternative equipment of the same design and operating principles of the same or of a different capacity.
b. Test Documentation
i. Chemistry Documentation:Application/compendial release requirements.
Notification of change and submission of updated batch records.
Stability testing: One batch on long-term stability.
ii. Dissolution Documentation
None beyond application/compendial release requirements.
iii. In Vivo Bioequivalence Documentation
None.
c. Filing Documentation : Annual report (long-term stability data).
2. Level 2 Changes
a. Definition of Level
Change in equipment to a different design and different operating principles.
b. Test Documentation
i. Chemistry Documentation
Application/compendial release requirements.
Notification of change and submission of updated batch records.
Stability testing:
Significant body of data available:One batch with three months accelerated stability data reported in
supplement; one batch on long-term stability data reported in annual report. Significant body of data not available: Up to three batches with three months accelerated stability data reported in supplement; up to three batches on long-term stability data reported in annual report.
ii. Dissolution Documentation:Case C dissolution profile.
iii. In Vivo Bioequivalence Documentation:None.
c. Filing Documentation
Prior approval supplement with justification for change; annual report (long-term stability data).
B. Process
1. Level 1 Changes
a. Definition of Level : This category includes process changes including changes such as mixing times and operating speeds within application/validation ranges.
b. Test Documentation
i. Chemistry Documentation:None beyond application/compendial release requirements.
ii. Dissolution Documentation:None beyond application/compendial release requirements.
iii. In Vivo Bioequivalence Documentation: None.
c. Filing Documentation:Annual report.
2. Level 2 Changes
a. Definition of Level:This category includes process changes including changes such as mixing times and operating speeds outside of application/validation ranges.
b. Test Documentation:
i. Chemistry Documentation
Application/compendial release requirements.
Notification of change and submission of updated batch records.
Stability testing: One batch on long-term stability.
ii. Dissolution Documentation:Case B dissolution profile.
iii. In Vivo Bioequivalence Documentation:None.
c. Filing Documentation:Changes being effected supplement; annual report (longterm stability data).
3. Level 3 Changes
a. Definition of Level:This category includes change in the type of process used in the manufacture of the product, such as a change from wet granulation to direct compression of dry powder.
b. Test Documentation
i. Chemistry Documentation
Application/compendial release requirements. Notification of change and submission of updated batch records.
Stability testing:
Significant body of data available:One batch with three months accelerated stability data reported in supplement; one batch on long-term stability data reported in annual report. Significant body of data not available:
Up to three batches with three months accelerated stability data reported in supplement; up to three batches on long-term stability data reported in annual report.
ii. Dissolution Documentation:Case B dissolution.
iii. In Vivo Bioequivalence Documentation: In vivo bioequivalence study. The bioequivalence study may be waived if a suitable in vivo/in vitro correlation has been verified.
c. Filing Documentation
Prior approval supplement with justification; annual report (long-term stability data).
IN VITRO DISSOLUTION
See current United States Pharmacopeia/National Formulary, section <711>, for general dissolution specifications.
IN VIVO BIOEQUIVALENCE STUDIES
Below is a general outline of an in vivo bioequivalence study. It is intended as a guide and the design of the actual study may vary depending on the drug and dosage form.
A. Objective:
To compare the rate and extent of absorption of the drug product for which the manufacture has been changed, as defined in this guidance, to the drug product manufactured prior to the change.
B. Design:
The study design should be a single dose, two-treatment, two-period crossover with adequate washout period between the two phases of the study. Equal numbers of subjects should be randomly assigned to each
of the two dosing sequences.
C. Selection of Subjects:
The number of subjects enrolled in the bioequivalence study should be determined statistically to account for the intrasubject variability and to meet the current bioequivalence interval.
D. Procedure:
Each subject should receive the following two treatments:
Treatment 1: Product manufactured with the proposed change.
Treatment 2: Product manufactured prior to the proposed change.
Following an overnight fast of at least 10 hours, subjects should receive either Treatments 1 or 2 above with 240 mL water. Food should not be allowed until 4 hours after dosing. Water may be allowed after the first
hour. Subjects should be served standardized meals beginning at 4 hours during the study.
E. Restrictions:
Prior to and during each study phase, water may be allowed ad libitum except for 1 hour before and after drug administration. The subject should be served standardized meals and beverages at specified times.
No alcohol or xanthine- or caffeine-containing foods and beverages should be consumed for 48 hours prior to each study period and until after the last blood sample is collected.
F. Blood Sampling:
Blood samples should be collected in sufficient volume for analysis of parent drug and active metabolite(s), if any. The sampling times should be such that it should be able to capture the Cmax and Tmax during the
absorption period. Sampling should be carried out for at least three terminal elimination half-lives for both parent drug and active metabolite(s). Whole blood, plasma or serum, whichever is appropriate for the analytes, should be harvested promptly and samples should be frozen at -20oC or -70oC to maintain sample stability.
G. Analytical Method:
The assay methodology selected should ensure specificity, accuracy, interday and intraday precision, linearity of standard curves, and adequate sensitivity, recovery, and stability of the samples under the storage and handling conditions associated with the analytical method.
H. Pharmacokinetic Analysis:
From the plasma drug concentration-time data, AUC0-t, AUC0-inf, Cmax, Tmax, Kel and t1/2 should be estimated.
I. Statistical Analysis:
Analysis of variance appropriate for a crossover design on the pharmacokinetic parameters using the general linear models procedures of SAS or an equivalent program should be performed, with examination of period, sequence and treatment effects. The 90% confidence intervals for the estimates of the difference between the test and reference least squares means for the pharmacokinetic parameters (AUC0-t, AUC0-inf, Cmax) should be calculated, using the two one-sided t-test procedure.
SUPAC-MR: Modified Release Solid Oral Dosage Forms
Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation
GENERAL STABILITY CONSIDERATIONS:
The effect SUPAC-type changes have on the stability of the drug product should be evaluated. For general guidance on conducting stability studies, applicants are referred to the FDA Guideline for Submitting Documentation for the Stability of Human Drugs and Biologics (02/87). For SUPAC submissions, the following points also should be considered:
· In most cases (except those involving scale up), stability data from pilot scale batches will be acceptable to support the proposed change.
· Where stability data show a trend toward potency loss or degradant increase under accelerated conditions, it is recommended that historical accelerated stability data from a representative prechange batch be submitted for comparison. It is also recommended that under these circumstances, all available long-term data on test batches from ongoing studies be provided in the supplement. Submission of historical accelerated and available long-term data would facilitate review and approval of the supplement.
· A commitment should be included to conduct long-term stability studies through the expiration dating period, according to the approved protocol, on the first or first three (see text for details) production batches and to report the results in the annual reports.
COMPONENTS AND COMPOSITION — NONRELEASE CONTROLLING EXCIPIENT
This section of the guidance focuses on changes in nonrelease controlling excipients in the drug product. For modified release solid oral dosage forms, consideration should be given as to whether the excipient is critical or not critical to drug release. The sponsor should provide appropriate justifications for claiming any excipient(s) as a nonrelease controlling excipient in the formulation of the modified release solid oral dosage form. The functionality of each excipient should be identified. Changes in the amount of the drug substance are not addressed by this guidance. Changes in components or composition that have the effect of adding a new excipient or deleting an excipient are defined at level 3 (defined below), except as described below in Section III.A.1.a. Waiver of bioequivalence testing for a change in composition which involves only a different color, flavor or preservative may be permissible as described in 21 CFR 320.22(d)(4).
A)Level 1 Change
Definition of Level:
Level 1 changes are those that are unlikely to have any detectable impact on formulation quality and performance.
Examples:
a. Deletion or partial deletion of an ingredient intended to affect the color or flavor of the drug product; or change in the ingredient of the printing ink to another approved ingredient.
b. Changes in nonrelease controlling excipients, expressed as percentage (w/w) of total formulation, less than or equal to the following percent ranges:
These percentages are based on the assumption that the drug substance in the product is formulated to 100% of label/potency. The total additive effect of all nonrelease controlling excipient changes should not be more than 5%. The total weight of the dosage form should still be within the original approved application range.
The components (active and excipients) in the formulation should have numerical targets that represent the nominal composition of the drug product on which any future changes in the composition of the product are to be based. Allowable changes in the composition should be based on the original approved target composition and not on previous level 1 changes in the composition. For products approved with only a range for excipients, the target value may be assumed to be the midpoint of the original approved application range.
2. Test Documentation
a. Chemistry documentation
Application/compendial product release requirements.
Stability: First production batch on long-term stability data reported in annual report.
b. Dissolution documentation:None beyond application/compendial requirements.
c. Bioequivalence documentation: None.
3. Filing Documentation: Annual report (all information including long-term stability data).
B. Level 2 Change
1)Definition of Level: Level 2 changes are those that could have a significant impact on formulation
quality and performance.
Examples:
a. A change in the technical grade and/or specifications of a nonrelease controlling excipient.
b. Changes in nonrelease controlling excipients, expressed as percentage (w/w) of total formulation, greater than those listed above for a level 1 change, but less than or equal to the following percent ranges (which represent a two-fold increase over level 1 changes):
These percentages are based on the assumption that the drug substance in the drug product is formulated to 100% of label/potency. The total additive effect of all nonrelease controlling excipient changes should not change by more than 10%. The total weight of the dosage form could still be within or outside the original approved application range.
The components (active and excipients) in the formulation should have numerical targets that represent the nominal composition of the product on which any future changes in the composition of the product are to be based. Allowable changes in the composition are based on the original approved target composition and not on the composition based on previous level 1 or level 2 changes. For products approved with only a range for excipients, the target value may be assumed to be the midpoint of the original approved application range.
2)Test documentation
a. Chemistry documentation:
Application/compendial product release requirements and updated executed batch records. Stability: One batch with three months accelerated stability data reported in prior approval supplement and long-term stability data of first production batch reported in annual report.
b. Dissolution documentation:
Extended release: In addition to application/compendial release requirements, multipoint dissolution profiles should be obtained in three other media, for example, in water, 0.1N HCl, and USP buffer media at pH 4.5, and 6.8 for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached. A surfactant may be used with appropriate justification
Delayed release: In addition to application/compendial release requirements, dissolution tests should be performed in 0.1 N HCl for 2 hours (acid stage) followed by testing in USP buffer media, in the range of pH 4.5-7.5 (buffer stage) under standard (application/compendial) test conditions and two additional agitation speeds using the application/
compendial test apparatus (three additional test conditions). If theapplication/compendial test apparatus is the rotating basket method (Apparatus 1), a rotation speed of 50, 100, and 150 rpm may be used, and if the application/compendial test apparatus is the rotating paddle method (Apparatus 2), a rotation speed of 50, 75, and 100 rpm may be used. Multipoint dissolution profiles should be obtained during the buffer stage of testing. Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached. The above dissolution testing should be performed using the changed drug product and the biobatch or marketed batch (unchanged drug product).
All modified release solid oral dosage forms: In the presence of an established in vitro/in vivo correlation (6), only application/compendial dissolution testing need be performed (i.e., only in vitro release data by the correlating method need to be submitted). The dissolution profiles of the changed drug product and the biobatch or marketed batch (unchanged drug product) should be similar. The sponsor should apply appropriate statistical testing with justifications (e.g., the f equation) for comparing 2 dissolution profiles . Similarity testing for the two dissolution profiles (i.e., for the unchanged drug product and the changed drug product) obtained in each individual medium is appropriate.
c. Bioequivalence documentation: none
3. Filing Documentation: Prior approval supplement (all information including accelerated stability data);
annual report (long-term stability data).
C. Level 3 Change
1. Definition of Level: Level 3 changes are those that are likely to have a significant impact on formulation quality and performance.
Example:Changes in the nonrelease controlling excipient range beyond those listed in Section III.B.1.b. The total weight of the dosage form may be within or outside the approved original application range
2. Test Documentation
a. Chemistry documentation:Application/compendial product release requirements and updated executed batch records.
Stability::Significant body of information available: One batch with three months accelerated stability data reported in prior approval supplement and longterm stability data of first three production batches reported in annual report. Significant body of information not available: Three batches with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b. Dissolution documentation:
Extended release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained using the application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 1, 2, and 4 hours and every two hours thereafter, until either 80% of the drug from the drug product is released or an asymptote is reached.
Delayed release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained during the buffer stage of testing using the application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached.
c. Bioequivalence documentation : A single-dose bioequivalence study . The bioequivalence study may be
waived in the presence of an established in vitro/in vivo correlation.
3. Filing Documentation: Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
COMPONENTS AND COMPOSITION — RELEASE CONTROLLING EXCIPIENT
This section of the guidance focuses on changes in release controlling excipients in the drug product. For modified release solid oral dosage forms, consideration should be given as to whether or not the excipient is critical to drug release. The sponsor should provide appropriate justifications (i.e., mechanism of drug release and manufacturing process) for claiming any excipient(s) as a release controlling excipient in the formulation of the modified release solid oral dosage form. The functionality of each excipient should be identified. Changes in the amount of the drug substance are not addressed by this guidance. Changes exceeding the ranges defined in each of the levels below may be allowed if considered to be within normal batch-to-batch variation and contained within an approved original application. In such situations, sponsors should contact the appropriate CDER review division for further guidance.
A) Level 1 Change:
1. Definition of Level: Level 1 changes are those that are unlikely to have any detectable impact on formulation quality and performance.
Example:
a. Changes in the release controlling excipient(s), expressed as percentage (w/w) of total release controlling excipient(s) in the formulation less than or equal to 5% w/w of total release controlling excipient content in the modified release solid oral dosage form.
The drug substance in the product is formulated to 100% of label/potency. The total additive effect of all release controlling excipient changes should not be more than 5% w/w of the total release controlling excipients in the original approved formulation. The total weight of the dosage form should still be within the approved original application range. The components (active and excipients) in the formulation should have numerical targets that represent the nominal composition of the product on which any future changes in the composition of the product are to be based. Allowable changes in the composition should be based on the original approved target composition and not on previous level 1 changes in the composition. For products approved with only a range for excipients, the target value may be assumed to be the midpoint of the original approved application range.
2. Test Documentation
a. Chemistry documentation: Application/compendial product release requirements.
Stability: First production batch on long-term stability data reported in annual report.
b. Dissolution documentation: None beyond application/compendial requirements.
c. Bioequivalence documentation: None.
3. Filing Documentation: Annual report (all information including long-term stability data).
B. Level 2 Change
1. Definition of Level: Level 2 changes are those that could have a significant impact on formulation quality and performance. Test documentation for a level 2 change would vary depending on whether the product could be considered to have a narrow therapeutic range.
Examples:
a. Change in the technical grade and/or specifications of the release controlling excipient(s).
b. Changes in the release controlling excipient(s), expressed as percentage (w/w) of total release controlling excipient(s) in the formulation, greater than those listed above for a level 1 change, but less than or equal to 10% w/w of total release controlling excipient content in the modified release solid oral dosage form.
The drug substance in the drug product is formulated to 100% of label/potency. The total additive effect of all release controlling excipient changes should not be more than 10% w/w of the total release controlling excipient(s) in the original approved formulation. The total weight of the dosage form could still be within or outside the approved original application range.
The components (active and excipients) in the formulation should have numerical targets that represent the nominal composition of the product on which any future changes in the composition of the product are to be based. Allowable changes in the composition are based on the original approved target composition and not on the composition based on previous level 1 or level 2 changes. For products approved with only a range for excipients, the target value may be assumed to be the midpoint of the original approved application range.
2. Test Documentation:
a) Chemistry documentation:Application/compendial product release requirements and updated executed batch records.
Stability:
Nonnarrow therapeutic range drugs: One batch with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first production batch reported in annual report.
Narrow therapeutic range drugs: Three batches with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b)Dissolution documentation:
Nonnarrow therapeutic range drugs:
Extended release: In addition to application/compendial release requirements, multipoint dissolution profiles should be obtained in three other media, for example, in water, 0.1N HCl, and USP buffer media at pH 4.5, and 6.8 for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached. A surfactant may be used with appropriate justification.
Delayed release: In addition to application/compendial release requirements, dissolution tests should be performed in 0.1 N HCl for 2 hours (acid stage) followed by testing in USP buffer media in the range of pH 4.5-7.5 (buffer stage) under standard (application/compendial) test conditions and two additional agitation speeds using the application/compendial test apparatus (three additional test conditions). If the application/compendial test apparatus is the rotating basket method (Apparatus 1), a rotation speed of 50, 100, and 150 rpm may be used, and if the application/compendial test apparatus is the rotating paddle method (Apparatus 2), a rotation speed of 50, 75, and 100 rpm may be used. Multipoint dissolution profiles should be obtained during the buffer stage
of testing. Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached. The above dissolution testing should be performed using the changed drug product and the biobatch or marketed batch (unchanged drug product). All modified release solid oral dosage forms: In the presence of an established in vitro/in vivo correlation, only application/compendial dissolution testing should be performed (i.e., only in vitro release data by the correlating method should be submitted). The dissolution profiles of the changed drug product and the biobatch or marketed batch (unchanged drug product) should be similar. The sponsor should apply appropriate statistical testing with justifications (e.g., the f equation) for comparing dissolution profiles . Similarity testing for the two dissolution profiles (i.e., for the unchanged drug product and the changed drug product) obtained in each individual medium is appropriate
Narrow therapeutic range drugs:
Extended release: In addition to application/compendial releaserequirements, a multipoint dissolution profile should be obtained in application/compendial medium for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or
an asymptote is reached. Delayed release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained during the buffer stage of testing using the application/compendial medium for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached
Delayed release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained during the buffer stage of testing using the application/compendial medium for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached.
c. Bioequivalence documentation
Nonnarrow therapeutic range drugs: None.
Narrow therapeutic range drugs: A single-dose bioequivalence study . The bioequivalence study may be waived in the presence of an established in vitro/in vivo correlation . Changes in release controlling excipients in the formulation should be within the range of release controlling excipients of the established correlation.
3. Filing Documentation: Prior approval supplement (all information including accelerated stability data);
annual report (long-term stability data).
C. Level 3 Change
1. Definition of Level: Level 3 changes are those that are likely to have a significant impact on formulation quality and performance affecting all therapeutic ranges of the drug.
Examples:
a. Addition or deletion of release controlling excipient(s) (e.g., release controlling polymer/plasticizer).
b. Changes in the release controlling excipient(s), expressed as percentage (w/w) of total release controlling excipient(s) in the formulation, greater than those listed above for a level 2 change (i.e., greater than 10% w/w of total release controlling excipient content in the modified release solid oral dosage form). Total weight of the dosage form may be within or outside the original approved application range.
2. Test Documentation:
a. Chemistry documentation:
Application/compendial product release requirements and updated executed batch records.
Stability: Three batches with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b. Dissolution documentation
Extended release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained using application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached.
Delayed release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained during the buffer stage of testing using the application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached.
c. Bioequivalence documentation: A single-dose bioequivalence study . The bioequivalence study may be waived in the presence of an established in vitro/in vivo correlation. Changes in release controlling excipients in the formulation should be within the range of release controlling excipients of the established correlation.
3. Filing Documentation: Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
V. SITE CHANGES:
Site changes consist of changes in location of the site of manufacture, packaging operations, and/or analytical testing laboratory for both company-owned and contract manufacturing facilities. They do not include any scale-up changes, changes in manufacturing (including process and/or equipment), or changes in components or composition. New manufacturing locations should have had a satisfactory current good manufacturing practice (cGMP) inspection. A stand-alone packaging operations site change, using container(s)/closure(s) in the approved application, may be submitted as a Changes Being Effected supplement. The facility should also have a current and satisfactory cGMP compliance profile with the FDA for the type of packaging operation in question before submitting the supplement. If the facility has not received a satisfactory cGMP inspection for the type of packaging operation in question, a prior approval supplement is recommended. The supplement should contain a written certification from the packaging facility stating that it is in conformance with cGMPs. It should also contain a commitment to place the first production batch of the product, and annual batches thereafter, on long-term stability studies using the approved protocol in the application and to submit the resulting data in annual reports. Where the product is available in more than one strength, size, or container/closure system, one lot of each combination should be placed on long-term stability studies. Bracketing or matrixing is allowed only if it has been approved previously by the FDA. Any changes to an approved stability protocol should have a supplemental approval prior to the initiation of the stability study.
A stand-alone analytical testing laboratory site change may be submitted as a Changes Being Effected supplement if the new facility has a current and satisfactory cGMP compliance profile with the FDA for the type of testing operation in question. The supplement should contain a commitment to use the same test methods employed in the approved application, written certification from the testing laboratory stating that they are in conformance with cGMPs, and a full description of the testing to be performed by the testing lab. If the facility has not received a satisfactory cGMP inspection for the type of testing involved, a prior approval supplement is recommended.
A. Level 1 Change:
1. Definition of Level: Level 1 changes consist of site changes within a single facility where the same equipment, standard operating procedures (SOPs), environmental conditions (e.g., temperature and humidity) and controls, and personnel common7 to both manufacturing sites are used and where no changes are made to the executed batch
records, except for administrative information and the location of the facility.
2. Test Documentation
a. Chemistry documentation: None beyond application/compendial product release requirements.
b. Dissolution documentation:None beyond application/compendial release requirements.
c. Bioequivalence documentation:None.
3. Filing Documentation: Annual report.
B. Level 2 Change:
1.Definition of Level: Level 2 changes consist of site changes within a contiguous campus, or between facilities in adjacent city blocks, where the same equipment, SOPs, environmental conditions
(e.g., temperature and humidity) and controls, and personnel common to both manufacturing sites are used and where no changes are made to the executed batch records, except for administrative information and the location of the facility.
2. Test Documentation
a. Chemistry documentation
Notification of location of new site and updated executed batch records. None beyond application/compendial product release requirements. Stability: One batch with three months accelerated stability data reported in Changes Being Effected supplement and long-term stability data of first production batch reported in annual report.
b. Dissolution documentation
Extended release: In addition to application/compendial release requirements, multipoint dissolution profiles should be obtained in three other media, for example, in water, 0.1N HCl, and USP buffer media at pH 4.5, and 6.8 for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached. A surfactant may be used with appropriate justification. Delayed release: In addition to application/compendial release requirements, dissolution tests should be performed in 0.1 N HCl for 2 hours (acid stage) followed by testing in USP buffer media, in the range of pH 4.5-7.5 (buffer stage) under standard (application/compendial) test conditions and two additional agitation speeds using the application/compendial test apparatus (three additional test conditions). If the application/compendial test apparatus is the rotating basket method (Apparatus 1), a rotation speed of 50, 100, and 150 rpm may be used, and if the application/compendial test apparatus is the rotating paddle method (Apparatus 2), a rotation speed of 50, 75, and 100 rpm may be used.
Multipoint dissolution profiles should be obtained during the buffer stage of testing. Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached. The above dissolution testing should be performed using the changed drug product and the biobatch or marketed batch (unchanged drug product). All modified release solid oral dosage forms: In the presence of an established in vitro/in vivo correlation (6), only application/compendial dissolution testing should be performed (i.e., only in vitro release data by the correlating method should be submitted). The dissolution profiles of the changed drug product and the biobatch or marketed batch (unchanged drug product) should be similar. The sponsor should apply appropriate statistical testing with justifications (e.g., the f equation) for comparing 2 dissolution profiles (5). Similarity testing for the two dissolution profiles (i.e., for the unchanged drug product and the changed drug product) obtained in each individual medium is appropriate.
c. Bioequivalence documentation: None.
3. Filing Documentation: Changes Being Effected supplement (all information including accelerated stability data); annual report (long-term stability data).
C. Level 3 Change
1. Definition of Level:Level 3 changes consist of a change in manufacturing site to a different campus. A different campus is defined as one that is not on the same original contiguous site or where the facilities are not in adjacent city blocks. To qualify as a level 3 change, the same equipment, SOPs, environmental conditions, and controls should
be used in the manufacturing process at the new site, and no changes may be made to the executed batch records except for administrative information, location and language translation, where needed.
2. Test Documentation
a. Chemistry documentation:Notification of location of new site and updated executed batch records. Application/compendial product release requirements.
Stability:
Significant body of information available: One batch with three months' accelerated stability data reported in prior approval supplement and longterm stability data of first three production batches reported in annual report.
Significant body of information not available: Three batches with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b. Dissolution documentation:
Extended release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained using application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached.
Delayed release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained during the buffer stage of testing using the application/compendial test conditions for
the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage
form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached.
c. Bioequivalence documentation: A single-dose bioequivalence study (3). The bioequivalence study may be
waived in the presence of an established in vitro/in vivo correlation.
3. Filing Documentation:
Prior approval supplement (all information including accelerated stability test data); annual report (long-term stability data).
VI. CHANGES IN BATCH SIZE (SCALE-UP/SCALE-DOWN)
Postapproval changes in the size of a batch from the pivotal/pilot scale biobatch material to larger or smaller production batches call for submission of additional information to the application. Scale-down below 100,000 dosage units is not covered by this guidance. Adjustments in parameters such as mixing times and speeds may be made to tailor the process to the characteristics of larger or smaller scale equipment. All scale-up changes should be properly validated and, where needed, inspected by appropriate Agency personnel.
A. Level 1 Change
1. Definition of Level
Change in batch size, up to and including a factor of ten times the size of the pilot/biobatch, where (1) the equipment used to produce the test batch(es) may vary in capacity, but are of the same design and operating principles; (2) the
batch(es) is manufactured in full compliance with cGMPs; and (3) the same standard operating procedures (SOPs) and controls, as well as the same formulation and manufacturing procedures, are used on the test batch(es) and on
the full-scale production batch(es).
2. Test Documentation
a. Chemistry documentation: Application/compendial product release requirements. Notification of change and submission of updated executed batch records in annual report.
Stability: First production batch on long-term stability data reported in annual report.
b. Dissolution documentation: None beyond application/compendial release requirements.
c. Bioequivalence documentation: None.
3. Filing Documentation:Annual report (all information including long-term stability data).
B. Level 2 Change
1. Definition of Level:Changes in batch size beyond a factor of ten times the size of the pilot/biobatch where (1) the equipment used to produce the test batch(es) is of the same design and operating principles; (2) the batch(es) is manufactured in full compliance with cGMPs; and (3) the same SOPs and controls as well as the same formulation and manufacturing procedures are used on the test batch(es) and on the full-scale production batch(es).
2. Test Documentation
a. Chemistry documentation
Application/compendial product release requirements. Notification of change and submission of updated batch
records.
Stability: One batch with three months' accelerated stability data reported in Changes Being Effected supplement and long-term stability data of first production batch reported in annual report.
b. Dissolution documentation
Extended release: In addition to application/compendial release requirements, multipoint dissolution profiles should be obtained in three other media, for example, in water, 0.1N HCl, and USP buffer media at pH 4.5, and 6.8 for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 1, 2, and 4 hours, and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached. A surfactant may be used with appropriate justification.
Delayed release: In addition to application/compendial release requirements, dissolution tests should be performed in 0.1 N HCl for 2 hours (acid stage) followed by testing in USP buffer media in the range of pH 4.5-7.5 (buffer stage) under standard (application/compendial) test conditions and two additional agitation speeds using the application/compendial test apparatus (three additional test conditions). If the application/compendial test apparatus is the rotating basket method (Apparatus 1), a rotation speed of 50, 100, and 150 rpm may be used, and if the application/compendial test apparatus is the rotating paddle method (Apparatus 2), a rotation speed of 50, 75, and 100 rpm may be used.
Multipoint dissolution profiles should be obtained during the buffer stage of testing. Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached. The above dissolution testing should be performed using the changed drug product and the biobatch or marketed batch (unchanged drug product). All modified release solid oral dosage forms: In the presence of an established in vitro/in vivo correlation (6), only application/compendial dissolution testing should be performed (i.e., only in vitro release data by the correlating method should be submitted). The dissolution profiles of the changed drug product and the biobatch or marketed batch (unchanged drug product) should be similar. The sponsor should apply appropriate statistical testing with justifications (e.g., the f equation) for comparing 2 dissolution profiles (5). Similarity testing for the two dissolution profiles (i.e., for the unchanged drug product and the changed drug product) obtained in each individual medium is appropriate.
3. Filing Documentation: sChanges Being Effected supplement (all information including accelerated stability data); annual report (long-term stability data).
VII. MANUFACTURING EQUIPMENT CHANGES
Manufacturing changes may involve the equipment used in the manufacturing process (critical manufacturing variable). If a manufacturer wishes to use manufacturing equipment that is not identical in every respect to the original manufacturing equipment used in the approved application, appropriate validation studies should be conducted to demonstrate that the new equipment is similar to the original equipment. For modified release solid oral dosage forms, consideration should be given as to whether or not the change in manufacturing equipment is
critical to drug release (critical equipment variable).
A. Level 1 Change:
1. Definition of Level:
This category consists of (1) change from nonautomated or nonmechanical equipment to automated or mechanical equipment to move ingredients and (2) change to alternative equipment of the same design and operating principles of the same or of a different capacity.
2. Test documentation
a. Chemistry documentation
Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Stability: First production batch on long-term stability data reported in annual report.
b. Dissolution documentation: None beyond application/compendial release requirements.
c. Bioequivalence documentation: None.
3. Filing Documentation
Annual report (all information including long-term stability data).
B. Level 2 Change
1. Definition of Level: Change in equipment to a different design and different operating principles.
2. Test Documentation:
a. Chemistry documentation
Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Stability:
Significant body of information available: One batch with three months' accelerated stability data reported in prior approval supplement and longterm stability data of first three production batches reported in annual report.
Significant body of information not available: Three batches with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b. Dissolution documentation
Extended release: In addition to application/compendial release requirements, multipoint dissolution profiles should be obtained in three other media, for example, in water, 0.1N HCl, and USP buffer media at pH 4.5, and 6.8 for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example, at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached. A surfactant may be used with appropriate justification.
Delayed release: In addition to application/compendial release requirements, dissolution tests should be performed in 0.1 N HCl for 2 hours (acid stage) followed by testing in USP buffer media, in the range of pH 4.5-7.5 (buffer stage) under standard (application/compendial) test conditions and two additional agitation speeds using the application/compendial test apparatus (three additional test conditions). If the application/compendial test apparatus is the rotating basket method (Apparatus 1), a rotation speed of 50, 100, and 150 rpm may be used, and if the application/compendial test apparatus is the rotating paddle method (Apparatus 2), a rotation speed of 50, 75, and 100 rpm may be used. Multipoint dissolution profiles should be obtained during the buffer stage of testing. Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached. The above dissolution testing should be performed using the changed drug product and the biobatch or marketed batch (unchanged drug product).
All modified release solid oral dosage forms: In the presence of an established in vitro/in vivo correlation (6), only application/compendial dissolution testing should be performed (i.e., only in vitro release data by the correlating method should be submitted). The dissolution profiles of the changed drug product and the biobatch or marketed batch (unchanged drug product) should be similar. The sponsor should apply appropriate statistical testing with justifications (e.g., the f equation) for comparing 2 dissolution profiles (5). Similarity testing for the two dissolution profiles (i.e., for the unchanged drug product and the changed drug product) obtained in each individual medium is appropriate.
c. Bioequivalence documentation: None.
3. Filing Documentation: Prior approval supplement with justification for change (all information including accelerated stability data); annual report (long-term stability data).
VIII. MANUFACTURING PROCESS CHANGES
Manufacturing changes may involve the manufacturing process itself (critical manufacturing variable). If a manufacturer wishes to use a manufacturing process that is not identical in every respect to the original manufacturing process used in the approved application, appropriate validation studies should be conducted to demonstrate that the new process is similar to the original process. For modified release solid oral dosage forms, consideration should be given as to whether or not the change in manufacturing process is critical to drug release (critical processing variable). For purposes of categorizing the level of changes, process change may be considered only to affect a release controlling excipient when both types of excipients (i.e.,nonrelease and release controlling) are present during the unit operation undergoing a change.
A. Level 1 Change
1. Definition of Level: Process changes involving adjustment of equipment operating conditions such as mixing times and operating speeds within original approved application ranges affecting the nonrelease controlling and/or release controlling excipient(s). The sponsor should provide appropriate justifications for claiming any excipient(s) as a nonrelease controlling or a release controlling excipient in the formulation of the modified release solid oral dosage form.
2. Test Documentation
a. Chemistry documentation:None beyond application/compendial product release requirements. Notification of the change and submission of the updated executed batch records.
b. Dissolution documentation:None beyond application/compendial release requirements.
c. Bioequivalence documentation:None.
3. Filing Documentation: Annual report.
B. Level 2 Change
1. Definition of Level
This category includes process changes involving adjustment of equipment operating conditions such as mixing times and operating speeds outside of original approved application ranges.
2. Test Documentation
a. Chemistry documentation
Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Stability: One batch with three months' accelerated stability data reported in Changes Being Effected supplement and long-term stability data of first production batch reported in annual report.
b. Dissolution documentation
Extended release: In addition to application/compendial release requirements, multipoint dissolution profiles should be obtained in three other media, for example, in water, 0.1N HCl, and USP buffer media at pH 4.5, and 6.8 for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached. A surfactant may be used with appropriate justification. Delayed release: In addition to application/compendial release requirements, dissolution tests should be performed in 0.1 N HCl for 2 hours (acid stage) followed by testing in USP buffer media, in the range of pH 4.5-7.5 (buffer stage) under standard (application/compendial) test conditions and two additional agitation speeds using the application/
compendial test apparatus (three additional test conditions). If the application/compendial test apparatus is the rotating basket method (Apparatus 1), a rotation speed of 50, 100, and 150 rpm may be used, and if the application/compendial test apparatus is the rotating paddle method (Apparatus 2), a rotation speed of 50, 75, and 100 rpm may be used. Multipoint dissolution profiles should be obtained during the buffer stage of testing. Adequate sampling should be performed, for example, at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached. The above dissolution testing should be performed using the changed drug product and the biobatch or marketed batch (unchanged drug product).
All modified release solid oral dosage forms: In the presence of an established in vitro/in vivo correlation (6), only application/compendial dissolution testing should be performed (i.e., only in vitro release data by the correlating method should be submitted). The dissolution profiles of the changed drug product and the biobatch or marketed batch (unchanged drug product) should be similar. The sponsor should apply appropriate statistical testing with justifications (e.g., the f equation) for comparing 2 dissolution profiles (5). Similarity testing for the two dissolution profiles (i.e., for the unchanged drug product and the changed drug product) obtained in each individual medium is appropriate.
c. Bioequivalence documentation: None.
3. Filing Documentation: Changes Being Effected supplement (all information including accelerated stability
data); annual report (long-term stability data).
C. Level 3 Change
1. Definition of Level:This category includes change in the type of process used in the manufacture of the product, such as a change from wet granulation to direct compression of dry powder.
2. Test Documentation
a. Chemistry documentation
Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Stability: Three batches with three months' accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b. Dissolution documentation:
Extended release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained using application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 1, 2, and 4 hours and every two hours thereafter until either 80% of the drug from the drug product is released or an asymptote is reached.
Delayed release: In addition to application/compendial release requirements, a multipoint dissolution profile should be obtained during the buffer stage of testing using the application/compendial test conditions for the changed drug product and the biobatch or marketed batch (unchanged drug product). Adequate sampling should be performed, for example at 15, 30, 45, 60, and 120 minutes (following the time from which the dosage form is placed in the buffer) until either 80% of the drug from the drug product is released or an asymptote is reached.
c. Bioequivalence documentation
A single-dose bioequivalence study. The bioequivalence study may be waived in the presence of an established in vitro/in vivo correlation .
3. Filing Documentation:Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
SUPAC-SS Nonsterile Semisolid Dosage Forms:
Chemistry, Manufacturing, and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation
I. INTRODUCTION
This guidance provides recommendations to pharmaceutical sponsors of new drug applications (NDAs), abbreviated new drug applications (ANDAs), and abbreviated antibiotic drug applications (AADAs) who intend to change (1) the components or composition, (2) the manufacturing (process and equipment), (3) the scale-up/scale-down of manufacture, and/or (4)the site of manufacture of a semisolid formulation during the postapproval period. This guidance addresses nonsterile semisolid preparations (e.g., creams, gels, lotions, and ointments) intended for topical routes of administration. The guidance defines (1) the levels of change; (2) recommended chemistry, manufacturing, and controls (CMC) tests to support each level of change; (3) recommended in vitro release tests and/or in vivo bioequivalence tests to support each level of change; and (4) documentation to support the change.
The guidance specifies the application information that should be provided to the Center for Drug Evaluation and Research (CDER) to ensure continuing product quality and performance chacteristics of the semisolid topical formulation for specified changes. The guidance does not comment on or otherwise affect compliance/inspection documentation defined by the Office of Compliance in CDER or the Office of Regulatory Affairs at FDA.
II. GENERAL BACKGROUND
In general, semisolid dosage forms are complex formulations having complex structural elements. Often they are composed of two phases (oil and water), one of which is a continuous (external) phase, and the other of which is a dispersed (internal) phase. The active ingredient is often dissolved in one phase, although occasionally the drug is not fully soluble in the system and is dispersed in one or both phases, thus creating a three-phase system. The physical properties of the dosage form depend upon various factors, including the size of the dispersed particles, the interfacial tension between the phases, the partition coefficient of the active ingredient between the phases, and the product rheology. These factors combine to determine the release characteristics of the drug, as well as other characteristics, such as viscosity.
A. Critical Manufacturing Parameters:
For a true solution, the order in which solutes are added to the solvent is usually unimportant. The same cannot be said for dispersed formulations, however, because dispersed matter can distribute differently depending on to which phase a particulate substance is added. In a typical manufacturing process, the critical points are generally the initial separation of a one-phase system into two phases and the point at which the active ingredient is added. Because the solubility of each added ingredient is important for determining whether a mixture is visually a single homogeneous phase, such data, possibly supported by optical microscopy, should usually be available for review. This is particularly important for solutes added to the formulation at a concentration near or exceeding that of their solubility at any temperature to which the product may be exposed. Variations in the manufacturing procedure that occur after either of these events are likely to be critical to the characteristics of the finished product. This is especially true of any process intended to increase the degree of dispersion through reducing droplet or particle size (e.g., homogenization). Aging of the finished bulk formulation prior to packaging is critical and should be specifically addressed in process validation studies.
B. General Stability Considerations:
The effect that SUPAC changes may have on the stability of the drug product should be evaluated. For general guidance on conducting stability studies, see the FDA Guideline for Submitting Documentation for the Stability of Human Drugs and Biologics. For SUPAC submissions, the following points should also be considered:
1. In most cases, except those involving scale-up, stability data from pilot scale batches will be acceptable to support the proposed change.
2. Where stability data show a trend towards potency loss or degradant increase under accelerated conditions, it is recommended that historical accelerated stability data from a representative prechange batch be submitted for comparison. It is also recommended that under these circumstances, all available long-term data on test batches from ongoing studies be provided in the supplement. Submission of historical accelerated and available long-term data would facilitate review and approval of the supplement.
3. A commitment should be included to conduct long-term stability studies through the expiration dating period, according to the approved protocol, on either the first or first three (see section III-VI for details) production batches, and to report the results in subsequent annual reports.
C. The Role of In Vitro Release Testing:
The key parameter for any drug product is its efficacy as demonstrated in controlled clinical trials. The time and expense associated with such trials make them unsuitable as routine quality control methods. Therefore, in vitro surrogate tests are often used to assure that product quality and performance are maintained over time and in the presence of change. A variety of physical and chemical tests commonly performed on semisolid products and their components (e.g., solubility, particle size and crystalline form of the active component, viscosity, and homogeneity of the product) have historically provided reasonable evidence of consistent performance. More recently, in vitro release testing has shown promise as a means to comprehensively assure consistent delivery of the active component(s) from semisolid products.
An in vitro release rate can reflect the combined effect of several physical and chemical parameters, including solubility and particle size of the active ingredient and rheological properties of the dosage form. In most cases, in vitro release rate is a useful test to assess product sameness between prechange and postchange products. However, there may be instances where it is not suitable for this purpose. In such cases, other physical and chemical tests to be used as measures of sameness should be proposed and discussed with the Agency. With any test, the metrics and statistical approaches to documentation of “sameness” in quality attributes should be considered.
The evidence available at this time for the in vitro-in vivo correlation of release tests for semisolid dosage forms is not as convincing as that for in vitro dissolution as a surrogate for in vivo bioavailability of solid oral dosage forms. Therefore, the Center’s current position concerning in vitro release testing is as follows:
1. In vitro release testing is a useful test to assess product “sameness” under certain scale-up and postapproval changes for semisolid products.
2. The development and validation of an in vitro release test are not required for approval of an NDA, ANDA or AADA nor is the in vitro release test required as a routine batch-to-batch quality control test.
3. In vitro release testing, alone, is not a surrogate test for in vivo bioavailability or bioequivalence.
4. The in vitro release rate should not be used for comparing different formulations across manufacturers.
III. COMPONENTS AND COMPOSITION:
This section of the guidance focuses on changes in excipients in the drug product. Qualitative changes in excipients should include only those excipients which are present in approved drug products for the specific route of administration. Quantitative changes in excipients should not exceed the amount previously approved in products with the same specific route of administration.2 The chronology of changes in components and composition should be provided. Changes in components or composition that have the effect of adding a new excipient or deleting an existing excipient are defined as level 3 changes (see section III.C below), except as described below. These changes generally result in the need to change the labeling.
Compositional changes in preservatives are considered separately and are not included as part of the total additive effect under sections III.A, B and C.
A. Level 1 Change
1. Definition of Level: Level 1 changes are those that are unlikely to have any detectable impact on formulation quality and performance.
Examples:
· Deletion or partial deletion of an ingredient intended to affect the color, fragrance, or flavor of the drug product.
· Any change in an excipient up to 5% of approved amount of that excipient. The total additive effect of all excipient changes should not be more than 5%. Changes in the composition should be based on the approved target composition and not on previous level 1 changes in the composition. A change in diluent (q.s. excipient) due to component and composition changes in excipient may be made and is excluded from the 5% change limit.
· Change in a supplier of a structure forming excipient that is primarily a single chemical entity (purity$95%) or change in a supplier or technical grade of any other excipient
2. Test Documentation
a. Chemistry Documentation: Application/compendial product release requirements and stability testing.
Stability testing: First production batch on long-term stability reported in annual report.
b. In Vitro Release Documentation: None.
c. In Vivo Bioequivalence Documentation: None
3. Filing Documentation
Annual report (all information including long-term stability data).
B. Level 2 Change
1. Definition of Level: Level 2 changes are those that could have a significant impact on formulation quality and performance.
Examples:
· Changes of >5% and #10% of approved amount of an individual excipient. The total additive effect of all excipient changes should not be more than 10%. Changes in the composition should be based on the approved target composition and not on previous level 1 or level 2 changes in the composition. Changes in diluent (q.s. excipient) due to component and composition changes in excipients are acceptable and are excluded from the 10% change limit.
· Change in supplier of a structure forming excipient not covered under level 1.
· Change in the technical grade of structure forming excipient.
· Change in particle size distribution of the drug substance, if the drug is in suspension.
2. Test Documentation
a. Chemistry Documentation: Application/compendial product release requirements and executed batch
records.
Stability testing: One batch with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first production batch reported in annual report.
b. In Vitro Release Documentation: The in vitro release rate of a lot of the new/modified formulation should be compared with that of a recent lot of comparable age of the pre-change formulation of the product. The median in vitro release rates (as estimated by the estimated slope from each cell, see section VII) of the two formulations should be demonstrated to be within acceptable limits using the testing procedure described in section VII (IN VITRO RELEASE TEST) below.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation: Changes being effected supplement (all information including accelerated stability data); annual report (long-term stability data).
C. Level 3 Change
1. Definition of Level: Level 3 changes are those that are likely to have a significant impact on formulation quality and performance.
Examples:
· Any qualitative and quantitative changes in an excipient beyond the ranges noted in level 2 change.
· Change in crystalline form of the drug substance, if the drug is in suspension.
2. Test Documentation
a. Chemistry Documentation: Application/compendial product release requirements and executed batch records. Significant body of information available: One batch with three months accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
Significant body of information not available: Three batches with three months accelerated stability data reported in prior approval supplement and long-term stability data of first three production batches reported in annual report.
b. In Vitro Release Documentation: The in vitro release rate of the new/modified formulation should be established as a point of reference. Under this level 3 change, in vitro release documentation is not required, but sponsors are encouraged to develop this information for use in subsequent changes under this guidance.
c. In Vivo Bioequivalence Documentation: Full bioequivalence study on the highest strength, with in vitro release/other approach on the lower strength(s).
3. Filing Documentation: Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
D. Preservative
For semisolid products, any change in the preservative may affect the quality of the product. If any quantitative or qualitative changes are made in the formulation, additional testing should be performed. No in vitro release documentation or in vivo bioequivalence documentation is needed for preservative changes.
1. Level 1 Change
a. Definition of Level: Quantitatively 10% or less change in the approved amount of preservative.
b. Test Documentation:
· Application/compendial product release requirements.
· Preservative Effectiveness Test carried out at lowest specified preservative level.
c. Filing Documentation: Annual report
2. Level 2 Change
a. Definition of Level: Quantitatively greater than 10% and up to 20% change in the approved amount of preservative.
b. Test Documentation:
· Application/compendial product release requirements.
· Preservative Effectiveness Test at lowest specified preservative level.
c. Filing Documentation:Changes being effected supplement.
3. Level 3 change
a. Definition of Level: Quantitatively greater than 20% change in the approved amount of preservative (including deletion) or use of a different preservative.
b. Test Documentation:
· Application/compendial product release requirements.
· Preservative Effectiveness Test at lowest specified preservative level.
· Analytical method for identification and assay for new preservative.
· Validation studies to show that the new preservative does not interfere with application/compendial test.
· Executed batch records.
· Stability testing: One batch with three months accelerated stability data reported in prior approval supplement and long-term stability data of first production batch reported in annual report.
c. Filing Documentation:
Prior approval supplement (all information including accelerated stability data); annual report (long-term stability data).
IV. MANUFACTURING
Manufacturing changes may affect both equipment used in the manufacturing process and the process itself.
A. Equipment
1. Level 1 Change:
a. Definition of Level :Change from nonautomated or nonmechanical equipment to automated or mechanical equipment to transfer ingredients. Change to alternative equipment of the same design and operating principles.
b. Test Documentation
i. Chemistry Documentation: Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Stability testing: First production batch on long-term stability reported in annual report.
ii. In Vitro Release Documentation:None.
iii. In Vivo Bioequivalence Documentation:None.
c. Filing Documentation: Annual report (all information including long-term stability data).
2. Level 2 Change:
a. Definition of Level: Change in equipment to a different design or different operating principles. Change in type of mixing equipment, such as high shear to low shear and vice versa.
b. Test Documentation:
i. Chemistry Documentation: Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Significant body of information available: One batch with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first production batch reported in annual report.
Significant body of information not available: Three batches with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first three production batches reported in annual report.
ii. In Vitro Release Documentation: The in vitro release rate of a lot of the dosage form prepared in new equipment should be compared with the release rate of a recent lot of comparable age of the product prepared using original equipment. The median in vitro release rates (as estimated by the estimated slope from each cell, see section VII) of the two formulations should be demonstrated to be within acceptable limits, using the testing procedure described in section VII (IN VITRO RELEASE TEST) below.
iii. In Vivo Bioequivalence Documentation: None
c. Filing Documentation: Changes being effected supplement (all information including accelerated stability data); annual report (long-term stability data).
3. Level 3 Change: No level 3 changes are anticipated in this category.
B. Process
1. Level 1 Change
a. Definition of Level: Process changes, including changes such as rate of mixing, mixing times, operating speeds, and holding times within approved application ranges. Also, order of addition of components (excluding actives) to either oil or water phase.
b. Test Documentation:
i. Chemistry Documentation: None beyond application/compendial product release requirements.
ii. In Vitro Release Documentation: None.
iii. In Vivo Bioequivalence Documentation: None.
c. Filing Documentation:Annual report.
2. Level 2 Change
a. Definition of Level:Change in equipment to a different design or different operating principles. Change in type of mixing equipment, such as high shear to low shear and vice versa.
b. Test Documentation:
i. Chemistry Documentation
Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Significant body of information available: One batch with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first production batch reported in annual report.
Significant body of information not available: Three batches with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first three
production batches reported in annual report.
ii. In Vitro Release Documentation:The in vitro release rate of a lot of the dosage form prepared in new equipment should be compared with the release rate of a recent lot of comparable age of the product prepared using original
equipment. The median in vitro release rates (as estimated by the estimated slope from each cell, see section VII) of the two formulations should be demonstrated to be within acceptable limits, using the testing procedure described in section VII (IN VITRO RELEASE TEST) below.
iii. In Vivo Bioequivalence Documentation:None.
c. Filing Documentation: Changes being effected supplement (all information including accelerated stability data); annual report (long-term stability data).
3. Level 3 Change: No level 3 changes are anticipated in this category.
V. BATCH SIZE (SCALE-UP/SCALE-DOWN)
This guidance recommends that the minimum batch size for the NDA pivotal clinical trial batch or the ANDA/AADA biobatch be at least 100 kg or 10% of a production batch, whichever is larger. Deviations from this recommendation should be discussed with the appropriate agency review division. All scale changes should be properly validated and may be inspected by appropriate agency personnel.
A. Level 1 Change
1. Definition of Level: Change in batch size, up to and including a factor of ten times the size of the pivotal clinical trial/biobatch, where: (1) the equipment used to produce the test batch(es) are of the same design and operating principles; (2) the batch(es) is manufactured in full compliance with cGMPs; and (3) the same standard operating
procedures (SOPs) and controls, as well as the same formulation and manufacturing procedures, are used on the test batch(es) and on the full-scale production batch(es).
2. Test Documentation:
a. Chemistry Documentation: Application/compendial product release requirements. Notification of
change and submission of updated executed batch records in annual report.
Stability testing: First production batch on long-term stability reported in annual report.
b. In Vitro Release Documentation :None.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation : Annual report (all information including long-term stability data).
B. Level 2 Change:
1. Definition of Level:Changes in batch size from beyond a factor of ten times the size of the pivotal clinical trial/biobatch, where: (1) the equipment used to produce the test batch(es) are of the same design and operating principles; (2) the batch(es) is manufactured in full compliance with cGMPs; and (3) the same standard operating procedures (SOPs) and controls, as well as the same formulation and manufacturing procedures, are used on the test batch(es) and on the full-scale production batch(es).
2. Test Documentation:
a. Chemistry Documentation: Application/compendial product release requirements. Notification of change and submission of updated executed batch records.
Stability testing: One batch with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first production batch reported in annual report.
b. In Vitro Release Documentation: The in vitro release rate of a lot of the scaled-up batch should be compared with the in vitro release rate of a recent lot, of comparable age, of the prechange scale. The median in vitro release rates (as estimated by the estimated slope from each cell, see section VII) of the lots of the two scales should be demonstrated to be within acceptable limits, using the testing procedure described in section VII (IN VITRO RELEASE TEST) below.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation: Changes being effected supplement (all information including accelerated stability data); annual report (long-term stability data).
C. Level 3 Change: No level 3 changes are anticipated in this category.
VI. MANUFACTURING SITE
Manufacturing site changes consist of changes in location in the site of manufacture, packaging/filling operations, and/or testing for both company owned and contract manufacturing facilities and do not include any other level 2 or 3 changes, e.g., changes in scale, manufacturing (including process and/or equipment), and components or composition. New manufacturing locations should have had a satisfactory cGMP inspection within the past two years. A stand-alone analytical testing laboratory site change may be submitted as a changes being effected supplement if the new facility has a current and satisfactory cGMP compliance profile with FDA for the type of testing operation in question. The supplement should contain a commitment to use the same test methods employed in the approved application, written certification from the testing laboratory stating that they are in conformance with cGMPs, and a full description of the testing to be performed by the testing lab. If the facility has not received a satisfactory cGMP inspection for the type of testing involved, a prior approval supplement is recommended. No stability data are needed for a change in a stand alone analytical facility.
A. Level 1 Change
1. Definition of Level: Level 1 changes consist of site changes within a single facility where the same equipment, standard operating procedures (SOPs), environmental conditions (e.g., temperature and humidity) and controls, and personnel common to both manufacturing sites are used, and where no changes are made to the manufacturing batch records, except for administrative information and the location of the facility. Common is defined as employees already working on the campus who have suitable experience with the manufacturing process.
2. Test Documentation:
a. Chemistry Documentation: None beyond application/compendial product release requirements.
b. In Vitro Release Documentation: None.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation: Annual report.
B. Level 2 Change
1. Definition of Level: Level 2 changes consist of site changes within a contiguous campus, or between facilities in adjacent city blocks, where similar equipment, standard operating procedures, (SOPs), environmental conditions (e.g., temperature and humidity) and controls, and personnel common to both manufacturing sites are used, and
where no changes are made to the manufacturing batch records, except for administrative information and the location of the facility.
2. Test Documentation:
a. Chemistry Documentation: Location of new site and updated executed batch records. None beyond application/compendial product release requirements.
Stability testing: First production batch on long-term stability reported in annual report.
b. In Vitro Release Documentation: None.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation:Changes being effected supplement; annual report (long-term stability data).
C. Level 3 Change
1. Definition of Level: Level 3 changes consist of a site change in manufacturing site to a different campus. A different campus is defined as one that is not on the same originalcontiguous site or where the facilities are not in adjacent city blocks. To qualify as a Level 3 change, similar equipment, SOPs, environmental conditions, and controls
should be used in the manufacturing process at the new site. Changes should not be made to the manufacturing batch records except when consistent with other level 1 changes. Administrative information, location, and language translation may be revised as needed. Any change to a new contract manufacturer also constitutes a level 3 change.
2. Test Documentation:
a. Chemistry Documentation
Location of new site and updated executed batch records. Application/compendial product release requirements.
Significant body of information available: One batch with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first three production batches reported in annual report.
Significant body of information not available: Three batches with three months accelerated stability data reported in changes being effected supplement and long-term stability data of first three production batches reported in annual report.
b. In Vitro Release Documentation: The in vitro release rate of a lot of the dosage form from the new manufacturing site should be compared with the in vitro release rate of a recent lot of comparable age of the dosage form manufactured at the prior site. The median in vitro release rates (as estimated by the estimated slope from each cell, see section VII) of the lots from the two sites should be demonstrated to be within acceptable limits, using the testing procedure described in section VII (IN VITRO RELEASE TEST) below.
c. In Vivo Bioequivalence Documentation: None.
3. Filing Documentation: Changes being effected supplement (all information including accelerated stability
data); annual report (long-term stability data).
VII. IN VITRO RELEASE TEST
In vitro release is one of several standard methods which can be used to characterize performance characteristics of a finished topical dosage form, i.e., semisolids such as creams, gels, and ointments. Important changes in the characteristics of a drug product formula or the thermodynamic properties of the drug(s) it contains should show up as a difference in drug release. Release is theoretically proportional to the square root of time (/t) when the formulation in question is in control of the release process because the release is from a receding boundary.
In vitro release method for topical dosage forms is based on an open chamber diffusion cell system such as a Franz cell system, fitted usually with a synthetic membrane. The test product is placed on the upper side of the membrane in the open donor chamber of the diffusion cell and a sampling fluid is placed on the other side of the membrane in a receptor cell. Diffusion of drug from the topical product to and across the membrane is monitored by assay of sequentially collected samples of the receptor fluid. The in vitro release methodology should be appropriately validated. Sample collection can be automated.
Aliquots removed from the receptor phase can be analyzed for drug content by high pressure liquid chromatography (HPLC) or other analytical methodology. A plot of the amount of drug released per unit area (mcg/cm2) against the square root of time yields a straight line, the slope of which represents the release rate. This release rate measure is formulation-specific and can be used to monitor product quality. The release rate of the biobatch or currently manufactured batch should be compared with the release rate of the product prepared after a change as defined in this guidance.
VIII. IN VIVO BIOEQUIVALENCE STUDIES:
The design of in vivo bioequivalence studies for semisolid dosage forms varies depending on the pharmacological activity of the drug and dosage form. A brief general discussion of such tests follows.
Objective:
To document the bioequivalence of the drug product for which the manufacture has been changed, as defined in this guidance, compared to the drug product manufactured prior to the change or compared to the reference listed drug (RLD).
Design:
The study design is dependent on the nature of the active drug. The bioequivalence study can be a comparative skin blanching study as in glucocorticoids (FDA, Topical Dermatological Corticosteroids: In Vivo Bioequivalence, June 2, 1995.) or a comparative clinical trial or any other appropriate validated bioequivalence study (e.g., dermatopharmacokinetic study) for the topical dermatological drug product.
Analytical Method:
The assay methodology selected should ensure specificity, accuracy, interday and intraday precision, linearity of standard curves, and adequate sensitivity, recovery, and stability of the samples under the storage and handling conditions associated with the analytical method.


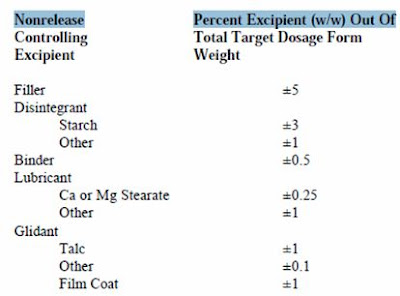


No comments:
Post a Comment